|
Why
does ice float? Ice
floats because it is less dense than water. Water has a density of 1.0 gm/cubic
cm. The density of ice Ih is 0.931 gm/cubic cm. But, why is ice less dense
than water if both are made up of molecules of H2O?
| [Ice
structure shown using the JMol Applet] | Try
this!! Click
the right mouse button with the cursor over the image--> Render
--> Schemes --> CPK Spacefill
Click on the left mouse button and rotate the ice structure. Do
you see the open spaces in ice? They are formed at low temperatures when water
molecules form many stable hydrogen bonds. Compare
this structure to that below of water at room temperature. |
| |
| [Water
Molecules are shown using the JMol Applet] | Render
--> Schemes --> CPK Spacefill Hydrogen
bond lengths between water molecules will vary since the molecules are in constant
motion, unlike ice which is a rigid lattice structure (above) To
measure distance Double
click on one atom then drag to second atom-- double click again
To
measure angles Double
click on first atom, once on middle atom, twice on atom three. |
| 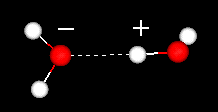
| Liquid
water has a partially ordered structure in which hydrogen
bonds are constantly being formed and breaking up. |
The strong hydrogen
bonds give water a high cohesiveness and, consequently, surface tension. This
is evident when small quantities of water are put onto a nonsoluble surface and
the water stays together as drops.
On
the other hand ice has a rigid lattice structure.
In liquid water each molecule is hydrogen bonded to approximately 3.4 other water
molecules. In
ice each each molecule is hydrogen bonded to 4 other molecules. Compare
the two structures below. Notice the empty spaces within the ice structure. In
ice Ih, each water forms four hydrogen bonds with O---O distances of 2.76 Angstroms
to the nearest oxygen neighbor. Because of ordered structure in ice there are
less H20 molecules in a given space of volume. Try
this -- 1)
Measure the O-O distances between any two adjacent oxygen atoms in ice shown in
the above structure.. Please
enter your answer in the space provided:
2) Measure the O-O-O
angle formed between adjacent oxygen atoms in ice.
3)
What is the length of the hydrogen bond H-O in ice?
| 
