ATP Molecule
The Adenosine triphosphate (ATP) molecule is the nucleotide known in biochemistry as the "molecular currency" of intracellular energy transfer; that is, ATP is able to store and transport chemical energy within cells. ATP also plays an important role in the synthesis of nucleic acids.
For 3-D Structure of this image using JsmolClick here
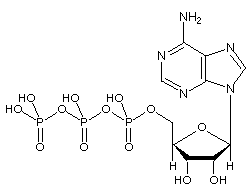
Image: Molecular Structure of ATP
Energy is released by hydrolysis of the third phosphate group. After this third phosphate group is released, the resulting ADP (adenosine diphosphate) can absorb energy and regain the group, thus regenerating an ATP molecule; this allows ATP to store energy like a rechargeable battery.
Phosphoryl positions
The phosphoryl groups starting with that on AMP are referred to as the alpha, beta, and gamma phosphates.
Physical and chemical properties
ATP consists of adenosine – composed of an adenine ring and a ribose sugar – and three phosphate groups (triphosphate). The phosphoryl groups, starting with the group closest to the ribose, are referred to as the alpha (α), beta (β), and gamma (γ) phosphates. Consequently, it is closely related to the adenine nucleotide, a monomer of RNA. ATP is highly soluble in water and is quite stable in solutions between pH 6.8 and 7.4, but is rapidly hydrolysed at extreme pH. Consequently, ATP is best stored as an anhydrous salt.
ATP is an unstable molecule in unbuffered water, in which it hydrolyses to ADP and phosphate. This is because the strength of the bonds between the phosphate groups in ATP is less than the strength of the hydrogen bonds (hydration bonds), between its products (ADP and phosphate), and water. Thus, if ATP and ADP are in chemical equilibrium in water, almost all of the ATP will eventually be converted to ADP. A system that is far from equilibrium contains Gibbs free energy, and is capable of doing work. Living cells maintain the ratio of ATP to ADP at a point ten orders of magnitude from equilibrium, with ATP concentrations fivefold higher than the concentration of ADP. This displacement from equilibrium means that the hydrolysis of ATP in the cell releases a large amount of free energy.
Two phosphoanhydride bonds (those that connect adjacent phosphates) in an ATP molecule are responsible for the high energy content of this molecule. In the context of biochemical reactions, these anhydride bonds are frequently – and sometimes controversially – referred to as high-energy bonds (despite the fact it takes energy to break bonds). Energy stored in ATP may be released upon hydrolysis of the anhydride bonds. The primary phosphate group on the ATP molecule that is hydrolyzed when energy is needed to drive anabolic reactions is the γ-phosphate group. Located the farthest from the ribose sugar, it has a higher energy of hydrolysis than either the α- or β-phosphate. The bonds formed after hydrolysis – or the phosphorylation of a residue by ATP – are lower in energy than the phosphoanhydride bonds of ATP. During enzyme-catalyzed hydrolysis of ATP or phosphorylation by ATP, the available free energy can be harnessed by a living system to do work.
Any unstable system of potentially reactive molecules could potentially serve as a way of storing free energy, if the cell maintained their concentration far from the equilibrium point of the reaction. However, as is the case with most polymeric biomolecules, the breakdown of RNA, DNA, and ATP into simpler monomers is driven by both energy-release and entropy-increase considerations, in both standard concentrations, and also those concentrations encountered within the cell.
The standard amount of energy released from hydrolysis of ATP can be calculated from the changes in energy under non-natural (standard) conditions, then correcting to biological concentrations. The net change in heat energy (enthalpy) at standard temperature and pressure of the decomposition of ATP into hydrated ADP and hydrated inorganic phosphate is −30.5 kJ/mol, with a change in free energy of 3.4 kJ/mol. The energy released by cleaving either a phosphate (Pi) or pyrophosphate (PPi) unit from ATP at standard state of 1 M are:
ATP + H
2O → ADP + Pi ΔG° = −30.5 kJ/mol (−7.3 kcal/mol)
ATP + H
2O → AMP + PPi ΔG° = −45.6 kJ/mol (−10.9 kcal/mol)
These values can be used to calculate the change in energy under physiological conditions and the cellular ATP/ADP ratio. However, a more representative value (which takes AMP into consideration) called the Energy charge is increasingly being employed. The values given for the Gibbs free energy for this reaction are dependent on a number of factors, including overall ionic strength and the presence of alkaline earth metal ions such as Mg2+
and Ca2+
. Under typical cellular conditions, ΔG is approximately −57 kJ/mol (−14 kcal/mol).
Synthesis
ATP can be produced by various cellular processes, most typically in mitochondria by oxidative phosphorylation under the catalytic influence of ATP synthase or in the case of plants in chloroplasts by photosynthesis.
The main fuels for ATP synthesis are glucose and fatty acids. Initially glucose is broken down into pyruvate in the cytosol. Two molecules of ATP are generated for each molecule of glucose. The terminal stages of ATP synthesis are carried out in the mitochondrion and can generate up to 34 ATP.
ATP in the human body
The total quantity of ATP in the human body is about 0.1 mole. The energy used daily by an adult calls for the hydrolysis of 200 to 300 moles of ATP. This means that each ATP molecule has to be recycled 2000 to 3000 times during the day. ATP cannot be stored and so its synthesis has to closely follow its consumption.
Other triphosphates
Living cells also have other "high-energy" nucleoside triphosphates, such as guanine triphosphate. Between them and ATP, energy can be easily transferred with reactions such as those catalyzed by nucleoside diphosphokinase: Energy is released when hydrolysis of the phosphate-phosphate bonds is carried out. This energy can be used by a variety of enzymes, motor proteins, and transport proteins to carry out the work of the cell. Also, the hydrolysis yields free inorganic phosphate and adenosine diphosphate, which can be broken down further to another phosphate ion and adenosine monophosphate. ATP can also be broken down to adenosine monophosphate directly, with the formation of pyrophosphate. This last reaction has the advantage of being an effectively irreversible process in aqueous solution.
Reaction of ADP with GTP
ADP + GTP > ATP + GDPMolecules of Life Resources
The Glucose Molecule
Glucose is the most important carbohydrates and is used as a source of energy in animals and plants. Glucose is also one of the main products of photosynthesis and starts respiration..