Hydrogen bonds in water -- Hydrogen bonds in Ice
What is a hydrogen bond?
A hydrogen bond is a type of attractive intermolecular force that exists between two partial electric charges of opposite polarity. Although stronger than most other intermolecular forces, the hydrogen bond is much weaker than both the ionic bond and the covalent bond.
As the name "hydrogen bond" implies, one part of the bond involves a hydrogen atom. The hydrogen must be attached to a strongly electronegative heteroatom, such as oxygen, nitrogen or fluorine, which is called the hydrogen-bond donor. This electronegative element attracts the electron cloud from around the hydrogen nucleus and, by decentralizing the cloud, leaves the atom with a positive partial charge. Because of the small size of hydrogen relative to other atoms and molecules, the resulting charge, though only partial, nevertheless represents a large charge density. A hydrogen bond results when this strong positive charge density attracts a lone pair of electrons on another heteroatom, which becomes the hydrogen-bond acceptor. For more on hydrogen bonds see: hydrogen bond
Hydrogen bonds in water
The most ubiquitous, and perhaps simplest, example of a hydrogen bond is found between water molecules. In a discrete water molecule, water has two hydrogen atoms and one oxygen atom. Two molecules of water can form a hydrogen bond between them. The oxygen of one water molecule has two lone pairs of electrons, each of which can form a hydrogen bond with hydrogens on two other water molecules.
The Water Dimer
The water dimer consists of two water molecules loosely bound by a hydrogen bond. It is the smallest water cluster. Because it is the simplest model system for studying hydrogen bonding in water, it has been the target of many theoretical studies on water dynamics.
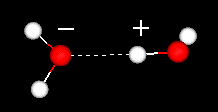 |
Two water molecules showing their hydrogen bond. For more details see: the water dimer. | |
|
The Water Dimer Try this: 1)Click spin off )Double click an oxygen of one of the molecules 4)Drag the mouse and double click a hydrogen along the hydrogen bond. What is the distance of the hydrogen bond in a water dimer in Angstrom (remember 10A = 1nm)?
|
The Case of H2O
Water can exist as a solid (ice), liquid (water liquid), or gas (water vapor). The basic molecular formula for the water molecule is the same in each H2O. But, as the temperature of the system changes the hydrogen bonds between water molecules change drastically.
In ice, the crystalline lattice is dominated by a regular array of hydrogen bonds which space the water molecules farther apart than they are in liquid water. This accounts for water's decrease in density upon freezing. In other words, the presence of hydrogen bonds enables ice to float, because this spacing causes ice to be less dense than liquid water.
Were the bond strengths more equivalent, one might instead find the atoms of two interacting water molecules partitioned into two polyatomic ions of opposite charge, specifically hydroxide and hydronium.(Hydronium ions are also known as 'hydroxonium' ions).
- H-O- H3O+
Indeed, in pure water under conditions of standard temperature and pressure, this latter formulation is applicable only rarely; on average about one in every 107 molecules gives up a proton to another water molecule, in accordance with the value of the dissociation constant for water under such conditions.
|
Hydrogen bonds in liquid water What is the hydrogen bond distance in water?
|
For an excellent technical read on hydrogen bonds in water see: Hydrogen Bonding in Water (1)
Hydrogen bonds in Ice
In ice Ih, each water forms four hydrogen bonds with O---O distances of 2.76 Angstroms to the nearest oxygen neighbor. The O-O-O angles are 109 degrees, typical of a tetrahedrally coordinated lattice structure. The density of ice Ih is 0.931 gm/cubic cm. This compares with a density of 1.00 gm/cubic cm. for water.
|
Hydrogen bonds in ice shown in green O-O distance in ice = 2.76 A HO hydrogen bond distance in ice is = 1.76 A Check these numbers out 1) Stop spin 2)Double click on one atom 3)Drag mouse then double click on 2nd atom |
Explain it with Molecules
- Why is water such a good solvent?
- Why does ice float?
- Why do solids, liquids and gases behave differently?
- What is the geometry of methane?
- What's the difference between alpha and beta glucose?
- How does caffeine work in the brain?
- How does soap work?
- What is the difference between sucrose and fructose?
- Why is carbon monoxide so dangerous?
- Why is graphite so soft if it is made of only carbon?
- What is the difference between Carbyne and Graphite?
- Why is the fullerene and similar structures the cornerstone of nanotechnology?
- How big is a nanotube?
- Why does table salt have a cubic crystal shape?
- What is the structure of the benzene molecule?
- Why do carcinogens cause cancer?
- What causes Sickle Cell Anemia?
- What is the difference between sodium nitrite and nitrate?
- How do drugs work?
World of Molecules
- The Periodic Table of Elements
- What is a Molecule?-- When is a Molecule also a Compound?
- 3D Structures using Jsmol/Jmol
- Explain it with Molecules
- Healthy Molecules from Exercise
- Acid and Base Molecules