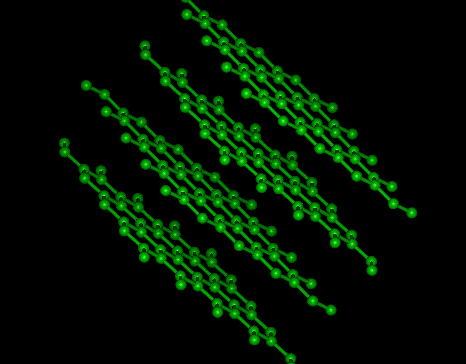
Graphite Molecular Structure
For 3-D Structure of Diamond Molecular Structure using Jsmol
Graphite is one of the allotropes of carbon. (See also allotropes of carbon.) Unlike diamond, graphite is a conductor, and can be used, for instance, as the material in the electrodes of an electrical arc lamp.
The pi orbital electrons delocalized across the hexagonal atomic sheets of carbon contribute the graphite's conductivity. In an oriented piece of graphite, conductivity parallel to these sheets is greater than that perpendicular to these sheets.
The loose coupling among the sheets in graphite contributes to another industrially important property -- graphite powder is used as a dry lubricant. Recent studies suggest that an effect called superlubricity can also account for this effect. Graphite is also used in pencils.
- Color is black silver.
- Luster is metallic to dull.
- Transparency crystals are opaque
- Crystal system is hexagonal; 6/m 2/m 2/m
- Crystal habits include massive lamellar veins and earthy masses, and scaly granules in metamorphic rocks.
- Hardness is 1 - 2
- Specific gravity is 2.2
- Cleavage is perfect in one direction.
- Fracture is flaky.
- Streak is black gray to brownish gray.
Associated minerals include quartz, calcite, micas, iron meteorites and tourmalines.
Other characteristics: thin flakes are flexible but inelastic, mineral can leave black marks on hands and paper, conducts electricity. In graphite the effect superlubricity also takes place
Best field indicators are softness, luster, density and streak.
- Diamond is hardest mineral known to man, but graphite is one of the softest.
- Diamond is an excellent electrical insulator, but graphite is a conductor of electricity.
- Diamond is the ultimate abrasive, but graphite is a very good lubricant.
- Diamond is usually transparent, but graphite is opaque.
- Diamond crystallizes in the isometric system but graphite crystallizes in the hexagonal system.
The unit cell dimensions are a = b = 2.456 Å, c = 6.694 Å. The carbon-carbon bond length in the bulk form is 1.418 Å, and the interlayer spacing is c/2 = 3.347 Å.