Fullerene Molecular Structure
For 3-D Structure of Fullerene Molecular Structure using Jsmol
Fullerenes, or buckminsterfullerenes in full, are molecules composed entirely of carbon, taking the form of a hollow sphere, ellipsoid, tube or ring.
Fullerenes are similar in structure to graphite, which is composed of a sheet of linked hexagonal rings, but they contain pentagonal (or sometimes heptagonal) rings that prevent the sheet from being planar. They are sometimes jocularly called buckyballs or buckytubes, depending on the shape. Cylindrical fullerenes are often called nanotubes. The smallest fullerene in which no two pentagons share an edge (which is destabilizing — see pentalene) is C60, and as such it is also the most common.
The molecule was named for Richard Buckminster Fuller, a noted architect who created the geodesic dome. Since buckminsterfullerenes have a similar shape to that sort of dome, the name was thought appropriate.
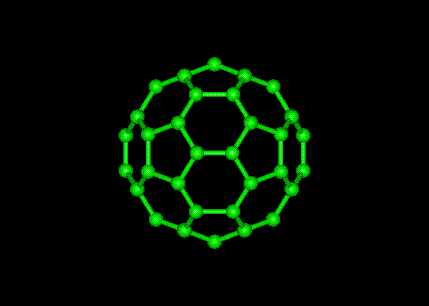
The structure of C60 is that of a truncated icosahedron, which resembles a football of the type made of hexagons and pentagons, with a carbon atom at the corners of each hexagon and a bond along each edge. A polymerized single-walled nanotubule (P-SWNT) is a substance composed of polymerized fullerenes in which carbon atoms from one buckytube bond with carbons in other buckytubes.
Until the late twentieth century, graphite and diamond were the only known allotropes of carbon. Then, in molecular beam experiments, discrete peaks were observed corresponding to molecules with the exact mass of 60, 70, or greater numbers of carbon atoms. Harold Kroto, from the University of Sussex, James Heath, Sean O'Brien, Robert Curl and Richard Smalley, from Rice University, discovered C60 and the fullerenes. Kroto, Curl, and Smalley were awarded the 1996 Nobel Prize in Chemistry for their roles in the discovery of this class of compounds. C60 and other fullerenes were later noticed occurring outside of a laboratory environment (e.g. in normal candle soot). By 1991 it was relatively easy to produce grams of fullerene powder using the techniques of Donald Huffman and Wolfgang Krätschmer. As of the early twenty-first century, the chemical and physical properties of fullerenes are still under heavy study, in both pure and applied research labs. In April 2003, fullerenes were under study for potential medicinal use — binding specific antibiotics to the structure to target resistant bacteria and even target certain cancer cells such as melanoma.
Fullerenes are not very reactive due to the stability of the graphite-like bonds, and are also fairly insoluble in many solvents. Researchers have been able to increase the reactivity by attaching active groups to the surfaces of fullerenes.
Other atoms can be trapped inside fullerenes, and indeed recent evidence for a meteor impact at the end of the Permian period was found by analysing noble gases so preserved.
Superconductivity is one of the more recently explored properties.
A common method used to produce fullerenes is to send a large current between two nearby graphite electrodes in an inert atmosphere. The resulting carbon plasma arc between the electrodes cools into sooty residue from which many fullerenes can be isolated.