Alanine Molecule
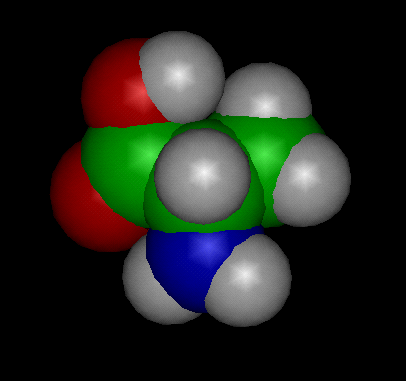
Image above: CPK (Space Filled Model) of the Alanine Molecule
Alanine (abbreviated as Ala or A; encoded by the codons GCU, GCC, GCA, and GCG) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain methyl group, classifying it as a nonpolar (at physiological pH), aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it.
For 3-D Structure of the Alanine Molecule using Jsmol Click here
Symbol: Ala A
Molecular Weight: 89.09
Isolectric point (pH) 6.0
Molecular Formula: C 3H7NO2
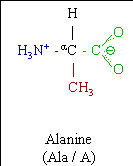
Alanine is one of the 20 most common natural amino acids. It is hydrophobic, with a methyl group side chain, and is the second-smallest of the 20 after glycine.
The α-carbon atom of the alanine molecule is bound to a methyl group (-CH3), making it one of the simplest α-amino acids and also results in alanine's classification as an aliphatic amino acid. The methyl group of alanine is non-reactive and is thus almost never directly involved in protein function.
Alanine is an amino acid molecule that cannot be phosphorylated, making it quite useful in a loss of function experiment with respect to phosphorylation.
Alanine is a non-essential amino acid that is
involved in the metabolism of tryptophan and the
vitamin pyridoxine. It is one of the most widely
used amino acids in protein construction, averaging
about 9 percent of average protein composition.
Alanine is found in prostate fluid, and may play
an important role in prostate health. Sources of
alanine are meat, poultry, eggs, dairy products,
and fish.