Butane Molecule
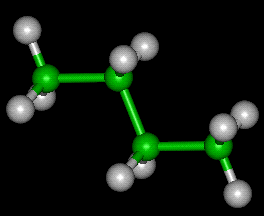
Butane, also called n-butane, is the unbranched alkane molecule with four carbon atoms, CH3CH2CH2CH3. Butane is also used as a collective term for n-butane together with its only other isomer, isobutane (also called methylpropane), CH(CH3)3.
To View the Butane Molecule in 3D --->>in 3D with Jsmol
Chemical and Physical Properties of Methane
When oxygen is plentiful, butane burns to form carbon dioxide and water vapor; when oxygen is limited, carbon (soot) or carbon monoxide may also be formed.
- 2C4H10 + 13O2 --> 8CO2 + 10H2O
Rotations about the central C-C bond producs two different conformations (trans and gauche) for n-butane.
Reactions and uses of Butane
Butanes are highly flammable, colorless, odorless, easily liquefied gases. The name butane was derived by back-formation from the name of butyric acid.
n-Butane is the feedstock for DuPont's catalytic process for the preparation of maleic anhydride:
- CH3CH2CH2CH3 + 3.5O2 → C2H2(CO)2O + 4H2O
n-Butane, like all hydrocarbons, undergoes free radical chlorination providing both 1-chloro- and 2-chlorobutanes, as well as more highly chlorinated derivatives. The relative rates of the chlorination is partially explained by the differing bond dissociation energies, 425 and 411 kJ/mol for the two types of C-H bonds. The two central carbon atoms have the slightly weaker C-H bonds.
Butane gas is sold bottled as a fuel for cooking and camping. When blended with Propane and other hydrocarbons, it is referred to commercially as LPG. It is also used as a petrol component, as a feedstock for the production of base petrochemicals in steam cracking, as fuel for cigarette lighters and as a propellant in aerosol sprays.
Very pure forms of butane, especially isobutane, can be used as refrigerants and have largely replaced the ozone layer depleting halomethanes, for instance in household refrigerators and freezers. The flammability of butane is not a major issue because the amount of butane in an appliance is not enough to cause a combustible mix given the amount of air in a room. The system operating pressure for butane is lower than for the halomethanes, such as R-12, so direct conversion of R-12 systems to butane, such as in automotive air conditioning systems, will not function optimally.
Butane used in Cooking Torches
Butane is currently the source of fuel for most cooking torches, A quick blast with a blowtorch is much quicker and easier than roasting in the oven for 20 minutes or putting under the broiler.--- Warming up knives to cut through frozen foods.--- Charring Corn And --Of course Marshmallows and Baked Alaska. See Cooking with a Blowtorch for more information.
Effects and health issues
Inhaling butane can cause drowsiness, narcosis, asphyxia; cardiac arrhythmia and frostbite, which can result in instant death from Asphyxiation, Acute toxicity and ventricular fibrillation. Butane is the most commonly misused volatile solvent in the UK, and was the cause of 52% of solvent related deaths in 2000.[1] By spraying butane directly into the throat, the jet of fluid can cool rapidly to –20 °C by expansion, causing prolonged laryngospasm.[2] "Sudden Sniffer's Death syndrome", first described by Bass in 1970,[3] is the most common single cause of solvent related death, resulting in 55% of known fatal cases.[2]
See also:
What is the geometry of the methane molecule? An interactive activity which includes a jmol applet of methane.
Readings and References
- Occupational and Safety Hazards for Butane CDC.GOV
- Ramsey J, Anderson HR, Bloor K, et al. An introduction to the practice, prevalence and chemical toxicology of volatile substance abuse. Hum Toxicol 1989
- Bass M. Sudden sniffing death. JAMA 1970
- Physical Properties for Butane
- Butane Molecule-- PubChem
- Cooking with a Butane Blowtorch