Estrogen Molecules
The three major naturally occurring estrogens in women are estrone (E1), estradiol (E2), and estriol (E3). Estradiol is the predominant estrogen during reproductive years both in terms of absolute serum levels as well as in terms of estrogenic activity.
To View the Estrogen Molecule in 3D --->>in 3D with Jsmol
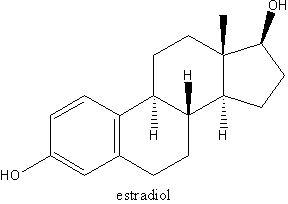
Estradiol molecular structure
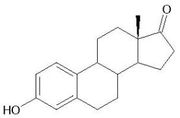
Estrone molecular structure
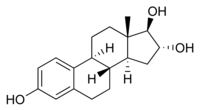
Estriol molecular structure
Chemical and Physical Properties of Estrogen
Estrogens (also oestrogens) are a group of steroid compounds, named for their importance in the oestrus cycle, functioning as the primary female sex hormone. While estrogens are present in both men and women, they are usually present at significantly higher levels in women of reproductive age. They promote the development of female secondary sex characteristics, such as breasts, and are also involved in the thickening of the endometrium and other aspects of regulating the menstrual cycle. Follicle stimulating hormone (FSH) and luteinizing hormone (LH) regulate the production of estrogen in ovulating women. Since estrogen circulating in the blood can feedback to reduce circulating levels of FSH and LH, some oral contraceptives contain estrogens.
The three major naturally occurring estrogens in women are estradiol, estriol and estrone. In the body these are all produced from androgens through enzyme action. Estradiol is produced from testosterone and estrone from androstenedione. Estrone is weaker than estradiol, and in post-menopausal women more estrone is present than estradiol.
Estrogen production
Estrogen is produced primarily by developing follicles in the ovaries, the corpus luteum and the placenta. Some estrogens are also produced in smaller amounts by other tissues such as liver, adrenal glands and the breasts. These secondary sources of estrogen are especially important in post-menopausal women. Synthesis of oestrogenes starts in theca interna cells in the ovary, by the synthesis of androstenedione from cholesterol. Androstenedione is a substance of moderate androgenic activity. This compound crosses the basal membrane into the surrounding granulosa cells, where it is converted to estrone or estradiol, either immediately or through testosterone. There is evidence that a testosterone supplement can support female sexual desire (Braunstein et al, 2005). Many of studies of the role of sex steroid hormones on sexual desire have been done in naturally post-menopausal women or women who have had their ovaries surgically removed. Such studies have found better correlation between sexual desire and androgen levels than for estrogen levels (Warnock et al, 2005). A clinical study found that women aged 18 to 44 who reported low sexual desire tended to have low levels of dehydroepiandrosterone sulfate (Davis et al, 2005). Dehydroepiandrosterone is an abundant sex steroid in women and like other steroids is efficiently sulfated. Dehydroepiandrosterone (DHEA) is a precursor steroid that can be converted to estrogens (estradiol) and androgens such as testosterone and 5a-dihydrotestosterone[1].
Medical applications
A range of synthetic and natural substances have been identified that possess estrogenic activity. These include bisphenol-A, phthalate esters and nonylphenol. The use of estrogenic compounds, especially together with a progestagen, is a treatment for the symptoms of menopause. Among the older postmenopausal women studied as part of the Women's Health Initiative (WHI), an orally-administered estogen supplement has been associated with an increased risk of dangerous blood clotting. The WHI studies used one type of estrogen supplement, a high oral dose of conjugated equine estrogens (Premarin alone and with Provera as Prempro)[2]. Research is underway to determine if risks of estrogen supplement use are the same for all estrogen supplement types. In particular, topically-applied estrogen may have a different spectrum of side-effects than does estrogen administered by the oral route[3].
Estrogen and lung disease
Among people over 70 who have never smoked, women make up 85 percent of those with chronic obstructive pulmonary disease (COPD). Mice studies suggest the possibility that COPD incidence may be tied to decreases in estrogen as women age. (Female mice that had their ovaries removed to deprive them of estrogen lost 45 percent of their working alveoli from their lungs. Upon receiving estrogen, the mice recovered full lung function.) Two proteins that are activated by estrogen play distinct roles in breathing. One protein builds new alveoli, the other stimulates the alveoli to expel carbon dioxide. Loss of estrogen hampered both functions in the test mice. (Massaro & Massaro, 2004)
References
- Stephen Nussey and Saffron Whitehead (2001).Metabolism of androgens to estradiol Endocrinology: An Integrated Approach, BIOS Scientific Publishers Ltd. ISBN 1859962521.
- Braunstein GD, Sundwall DA, Katz M, Shifren JL, Buster JE, Simon JA, Bachman G, Aguirre OA, Lucas JD, Rodenberg C, Buch A, Watts NB. (2005). "Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial". Archives of Internal Medicine 165 (14): 1582-9. PMID 16043675.
- Davis SR, Davison SL, Donath S, Bell RJ. (2005). "Circulating androgen levels and self-reported sexual function in women". Journal of the American Medical Association 294 (1): 91-6. (Full text requires registration)
- Massaro D, Massaro GD (2004). "Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice". American Journal of Physiology. Lung Cellular and Molecular Physiology 287 (6): L1154-9. PMID 15298854.
- Warnock JK, Swanson SG, Borel RW, Zipfel LM, Brennan JJ. (2005). "Combined esterified estrogens and methyltestosterone versus esterified estrogens alone in the treatment of loss of sexual interest in surgically menopausal women". Menopause 12 (4): 374-84. PMID 16037752.