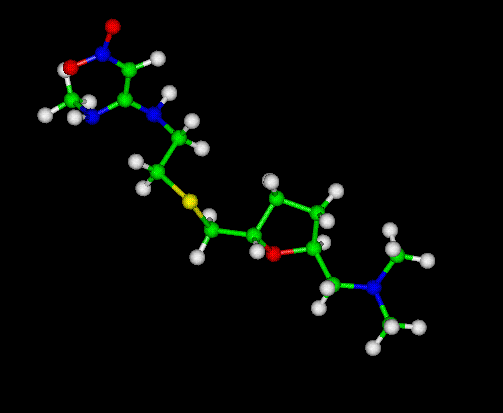
The Zantac Molecule --Ranitidine
Zantac -- Ranitidine
Ranitidine is a histamine H2-receptor antagonist that inhibits stomach acid production. It is commonly used in the treatment of peptic ulcer disease (PUD) and gastroesophageal reflux disease (GERD). It is currently marketed over the counter under the trade name Zinetac and Zantac by GlaxoSmithKline and by many other companies under various other names.
Clinical Use
Certain preparations of ranitidine are available over the counter (OTC) in various countries. In the United States, 75 mg and 150 mg tablets are available OTC. In Australia, packs containing 7 or 14 doses of the 150 mg tablet are available in supermarkets, small packs of 150 mg and 300 mg tablets are Schedule 2 Pharmacy Medicines. Larger doses and pack sizes still require a prescription. Outside of the United States, ranitidine is combined with bismuth (which acts as a mild antibiotic) as a citrate salt (ranitidine bismuth citrate, Tritec®), to treat Helicobacter pylori infections. This combination is usually given with clarithromycin, another antibiotic.
History and development
Ranitidine was developed by Glaxo (now GlaxoSmithKline) in an effort to match the success of Smith, Kline & French (also now GlaxoSmithKline) with the first histamine H2-receptor antagonist cimetidine. Ranitidine was the result of a rational drug-design process using what was by then a fairly refined model of the histamine H2-receptor and quantitative structure-activity relationships (QSAR). Glaxo refined the model further by replacing the imidazole-ring of cimetidine with a furan-ring with a nitrogen-containing substituent, and in doing so developed ranitidine. Ranitidine was found to have a far-improved tolerability profile (i.e. fewer adverse drug reactions), longer-lasting action, and ten times the activity of cimetidine. Ranitidine was introduced in 1981 and was the world's biggest-selling prescription drug by 1988. It has since largely been superseded by the even more effective proton pump inhibitors, with omeprazole becoming the biggest-selling drug for many years.
Following contents from: From LiverTox - NIH (National Institute of Health)
Introduction
Ranitidine is a histamine type 2 receptor antagonist (H2 blocker) which is widely used for treatment of acid-peptic disease and heartburn. Ranitidine has been linked to rare instances of clinically apparent acute liver injury.Background
Ranitidine (ra ni' ti deen) was the second H2 blocker introduced into clinical practice in the United States and remains a commonly used agent for treatment of duodenal and gastric ulcer and gastroesophageal reflux disease. The H2 blockers are specific antagonists of the histamine type 2 receptor, which is found on the basolateral (antiluminal) membrane of gastric parietal cells. The binding of ranitidine to the H2 receptor results in inhibition of acid production and secretion, and improvement in symptoms and signs of acid-peptic disease. The H2 blockers inhibit an early, “upstream” step in gastric acid production and are less potent that the proton pump inhibitors, which inhibit the final common step in acid secretion. Nevertheless, the H2 blockers inhibit 24 hour gastric acid production by about 70% and are most effective in blocking basal and nocturnal acid production. Ranitidine was first approved for use in the United States in 1983 and is now used widely both by prescription and over-the-counter. The listed indications for ranitidine are duodenal and gastric ulcer disease, gastroesophageal reflux and prevention of stress ulcers. Ranitidine is available by prescription in capsules of 150 and 300 mg in several generic forms, and in both oral and parenteral forms under the brand name Zantac. Over-the-counter formulations of ranitidine are usually tablets of 75 mg each. The typical recommended dose of ranitidine for therapy of peptic ulcer disease in adults is 150 mg twice daily or 300 mg once nightly for 4 to 8 weeks, and maintenance doses of 150 mg once daily. Lower, chronic or intermittent doses are used for therapy of heartburn and indigestion. Side effects of ranitidine are uncommon, usually minor, and include diarrhea, constipation, fatigue, drowsiness, headache and muscle aches. Ranitidine is metabolized by, but minimally affects the activity of the hepatic cytochrome P450 enzymes, for which reason it is less likely to lead to drug-drug interactions than is cimetidine.Hepatotoxicity
Chronic therapy with ranitidine has been associated with minor elevations in serum aminotransferase levels in 1% to 4% of patients, but similar rates were reported in placebo recipients. The ALT elevations are usually asymptomatic and transient and may resolve without dose modification. Rare instances of clinically apparent liver injury have been reported in patients receiving ranitidine, but the time to onset and pattern of injury has varied greatly (Cases 1-3). Onset can be as short as a few days to as long as several months, but is usually within 6 weeks. The pattern of serum enzyme elevation varies from hepatocellular to cholestatic, most cases being “mixed” hepatocellular-cholestatic. The injury is rarely severe and usually resolves rapidly upon stopping, generally within 4 to 12 weeks. Liver biopsy histology often shows prominent centrolobular necrosis. Immunoallergic features (rash, fever, eosinophilia) can occur (Case 2) but are uncommon, as is autoantibody formation.Mechanism of Injury
Ranitidine is metabolized by the microsomal P450 drug metabolizing enzymes and inhibits the function of CYP 3A and 2D6, and injury may be the result of its activation to a toxic intermediate. Rapid recurrence with rechallenge is typical, but features of hypersensitivity are uncommon.
Outcome and Management
The hepatic injury caused by ranitidine is usually rapidly reversible with stopping the medication (Case 1). Rare instances of acute liver failure have been attributed to it, but ranitidine has not been definitively linked to cases of prolonged cholestasis or vanishing bile duct syndrome. Rechallenge usually causes recurrence and should be avoided. Interestingly, there appears to be little cross reactivity in hepatic injury between ranitidine and cimetidine. If acid suppression is required, use of an unrelated proton pump inhibitor is probably prudent for patients with clinically apparent ranitidine induced liver injury.The H2 receptor blockers include cimetidine, famotidine, nizatidine, and ranitidine. General references on all four agents are given together after the overview section on H2 Blockers, while specific references are provided after the description of each drug. See also the Proton Pump Inhibitors.
External links
- [1] - Consumer information on Zantac from the manufacturer.
- Zantac Research - Current research on Zantac from the primary literature.